Abstract
Introduction: Most patients (pts) with newly diagnosed classical Hodgkin lymphoma (cHL) are cured by multi-agent chemotherapy (after risk stratification), with or without radiotherapy. Standard of care for relapsed/refractory cHL is salvage chemotherapy, followed by autologous hematopoietic cell transplantation (auto-HCT) if chemosensitive, but for pts who fail auto-HCT, outcomes are generally poor. Recently, programmed death-1 (PD-1) inhibitors such as nivolumab and pembrolizumab, and the antibody-drug conjugate brentuximab vedotin (BV), have been approved for such pts. Our understanding of how to use these novel agents to optimize outcomes and quality of life (QoL) while minimizing toxicity is rapidly evolving; however, there are limited data describing efficacy and safety in clinical practice. Similarly, limited data exist on the significance of molecular features of tumors and pts. This ongoing, observational, multicenter, prospective study (NCT02856646) investigates current cHL treatment patterns in real-world clinical practice, assesses pt QoL, and explores how tumor/pt molecular characteristics affect outcomes under different treatment modalities. Here we present initial interim data.
Methods: Eligible pts were ≥18 y old with histologically confirmed cHL. At enrollment, pts were treatment naïve or within 2 wk of beginning any line of anticancer therapy. Index therapy was defined as therapy received at or within 2 wk of enrollment. Data from US oncology practices were collected from pt medical records via case report forms, pt-reported outcome questionnaires, and tissue samples from routine testing (planned up to 5 y). The coprimary objectives are to describe current treatment patterns and to evaluate clinical outcomes in clinical practice by pt subgroups, with a target enrollment of 500 pts. Additional objectives include QoL assessment and characterization of tumor and pt immunophenotypic, genomic, and molecular profiles (data not shown).
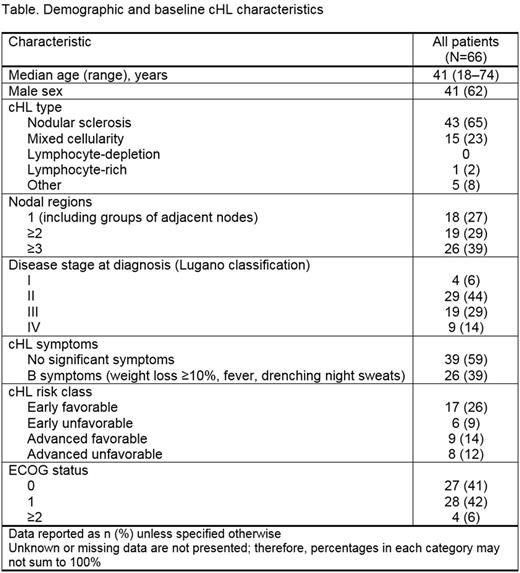
Results: Between study start (October 2016) and data cut-off, 66 pts were enrolled with a 4.1-mo median follow-up. The Table shows demographic and baseline cHL characteristics. At enrollment, 76% of pts were treatment naïve and 24% had received prior therapy. Of the 15 pts previously treated with systemic therapy, 60%, 20%, and 13% had received 1st, 2nd, and 3rd lines of therapy, respectively. The most frequent systemic therapies prior to enrollment were doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; 93%); BV (33%); and ifosfamide, carboplatin, and etoposide (ICE; 27%). In pts who discontinued prior treatment, the most common causes were: therapy completion (73%), relapse/progression (33%), and remission (13%). At enrollment, index treatment types were immunotherapy (6% of pts, all of whom remained on immunotherapy) and chemotherapy (89% remained on index chemotherapy; 5% switched to non-chemotherapy). The most common index therapies were ABVD (71%) and ICE (12%). In total, 80%, 3%, and 9% of index therapies were induction, maintenance, and consolidation/intensification regimens, respectively. Complete response was achieved in 83% of pts who had completed index therapy. Of pts who discontinued index therapy, 50%, 25%, and 25% discontinued due to toxicity, progression, and therapy completion, respectively. All-grade treatment-related adverse events (TRAEs) occurred in 61% of all pts; AEs in ≥10% of pts were fatigue (18%), nausea (18%), neutropenia (17%), and constipation (15%). Concomitant medication for TRAEs was received by 44% of pts, with a mean (median) of 4 (3) agents per pt. Analyses of QoL, prevalence of 9p24.1 amplification, correlation of PD-1 ligand expression with clinical outcomes, and additional molecular profiling are ongoing.
Conclusions: To our knowledge, this is the first prospective registry study to assess treatment patterns and outcomes including QoL in cHL. Initial data suggest that most pts receive multi-agent chemotherapy as 1st-line treatment, consistent with the current treatment paradigm. Analyses by pt subgroup and evaluation of novel agents for 2nd-line treatment and beyond will continue as enrollment accrues. Long-term focuses of this study include pt-reported outcomes and characterization of tumor/pt biomarkers in relation to clinical outcomes. This work may help individualize therapy for pts with cHL.
Study support: BMS. Writing support: L Yee, Caudex, funded by BMS.
Yasenchak: Seattle Genetics: Consultancy; Bristol-Myers Squibb: Consultancy. Svoboda: BMS: Consultancy, Research Funding; Kite: Consultancy; Seattle Genetics: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Celgene: Research Funding. Stevens: Bayer: Membership on an entity's Board of Directors or advisory committees. Gajavelli: Bristol-Myers Squibb: Employment. Le: Bristol-Myers Squibb: Employment. Armand: Sigma Tau: Research Funding; Otsuka: Research Funding; Infinity: Consultancy; Sequenta/Adaptive: Research Funding; Pfizer: Consultancy, Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Tensha: Research Funding; Affimed: Research Funding; Roche: Research Funding; Genmab: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal